Microcystins are a group of cyclic heptapeptide (7 amino acids) hepatotoxins
(liver toxins) produced by a number of cyanobacterial genera, the most notable of which is the widespread Microcystis from which the toxins take their name. Microcystins have been reported in this organism and other cyanobacteria world- wide. There have been approximately 60 different microcystins identified to date.
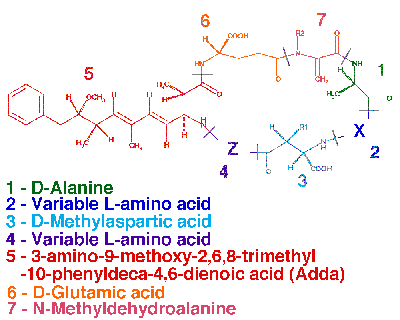
Microcystins consist of a seven-membered peptide ring which is made up of five non-protein amino acids and two protein amino acids. It is these two protein amino acids that distinguish microcystins from one another, while the other amino acids are more or less constant between variant microcystins. Using amino acid single letter code nomenclature, each microcystin is designated a name depending on the variable amino acids which complete their structure. The most common and potently toxic microcystin-LR contains the amino acids Leucine (L) and Arginine (R) in these variable positions.
Below is the general stucture of microcystins showing the variable amino acid positions "X" and "Z". The amino acids are delineated in this diagram and numbered according to the microcystin standard nomenclature. R1 and R2 are H in demethylated microcystins.
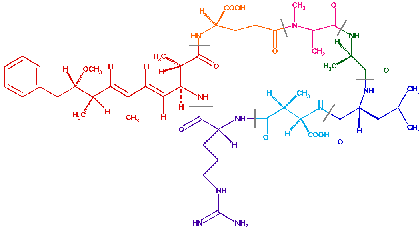
The molecular structure of microcystin-LR can be seen below, with the variable amino acids leucine and arginine in Blue and Purple respectively.
View an animation of MCYST-LR.
The "Adda" amino acid has become a useful tool in microcystin research as it provides the molecule with a characterstic wavelength absorbance at 238 nm. This is believed to be attributable to the conjugated diene group in the long carbon chain of this uncommon amino acid. The Adda moiety is also required for toxicity and is important in the binding of the toxin to protein phosphatases. The stereochemistry about the dienes of the Adda group have also been shown to influence toxicity, as too have the levels of methylation of various structures in the cyclic peptide. As such, the relative toxicities of microcystins can differ greatly. The absorbance characteristics of Adda provide a means of analysis of microcystins after separating them by reverse phase HPLC.

